- Industry Solutions
Industry Solutions
From reshaping the quote to cash process, to transforming engagement with channels partners, to achieving excellence in global product launch, Model N enables digital reinvention with industry-specific solutions that maximize revenue.
- Right block
- Industry Solutions
Industry Solutions
From reshaping the quote to cash process, to transforming engagement with channels partners, to achieving excellence in global product launch, Model N enables digital reinvention with industry-specific solutions that maximize revenue.
- Right block
- Products
Our Products
Model N delivers a platform for business reinvention, empowering companies to maximize revenue as they transform Sales, Marketing, Channels, Finance, and Legal processes.
- Right block
- Services & Support
Services & Support
To ensure you get the most out of your Model N investment Model N provides a complete set of professional Customer Success, Services and Support offerings designed to further your business and IT success.
- Right block
- Resources
- Resources
Webinar: Closing Medtech’s Great Divide
Register for this 30 minute webinar today!
Discover how you can boost operational efficiency by closing the gap between your front and back office.
- Right block
- Resources
- Company
Company
Model N supports the complex business needs of the world’s leading brands in pharmaceutical, medical device, high tech, manufacturing and semiconductors across more than 120 countries, including Pfizer, AstraZeneca, Sanofi, Gilead, Abbott, Stryker, AMD, Micron, Seagate, STMicroelectronics, NXP, Sesotec, and Southern States.
- Right block
Contact Sales

 Whitepaper
Whitepaper






 webinar
webinar

 case-studies
case-studies


 whitepaper
whitepaper
.
- Products
Our Products
Model N delivers a platform for business reinvention, empowering companies to maximize revenue as they transform Sales, Marketing, Channels, Finance, and Legal processes.
- Right block
- Services & Support
Services & Support
To ensure you get the most out of your Model N investment Model N provides a complete set of professional Customer Success, Services and Support offerings designed to further your business and IT success.
- Right block
- Resources
- Resources
Webinar: Closing Medtech’s Great Divide
Register for this 30 minute webinar today!
Discover how you can boost operational efficiency by closing the gap between your front and back office.
- Right block
- Resources
- Company
Company
Model N supports the complex business needs of the world’s leading brands in pharmaceutical, medical device, high tech, manufacturing and semiconductors across more than 120 countries, including Pfizer, AstraZeneca, Sanofi, Gilead, Abbott, Stryker, AMD, Micron, Seagate, STMicroelectronics, NXP, Sesotec, and Southern States.
- Right block
- Industry Solutions
Industry Solutions
From reshaping the quote to cash process, to transforming engagement with channels partners, to achieving excellence in global product launch, Model N enables digital reinvention with industry-specific solutions that maximize revenue.
- Right block
- Products
Our Products
Model N delivers a platform for business reinvention, empowering companies to maximize revenue as they transform Sales, Marketing, Channels, Finance, and Legal processes.
- Right block
- Services & Support
Services & Support
To ensure you get the most out of your Model N investment Model N provides a complete set of professional Customer Success, Services and Support offerings designed to further your business and IT success.
- Right block
- Resources
- Resources
Webinar: Closing Medtech’s Great Divide
Register for this 30 minute webinar today!
Discover how you can boost operational efficiency by closing the gap between your front and back office.
- Right block
- Resources
- Company
Company
Model N supports the complex business needs of the world’s leading brands in pharmaceutical, medical device, high tech, manufacturing and semiconductors across more than 120 countries, including Pfizer, AstraZeneca, Sanofi, Gilead, Abbott, Stryker, AMD, Micron, Seagate, STMicroelectronics, NXP, Sesotec, and Southern States.
- Right block
Quick Links


Fill out the form to download this whitepaper
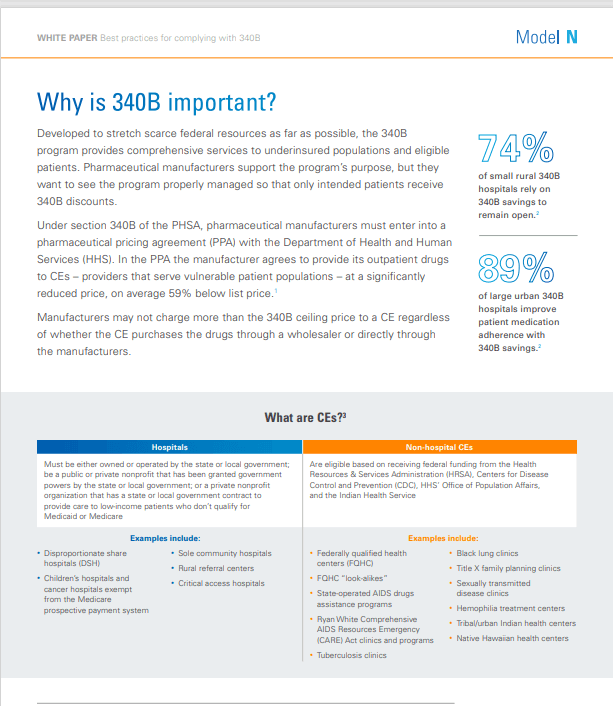
The Public Health Service Act 340B Drug Pricing Program enables providers that serve vulnerable patient populations to access outpatient drugs at significantly lower costs, which helps improve overall patient care. But for pharmaceutical manufacturers, program management can be extremely challenging.


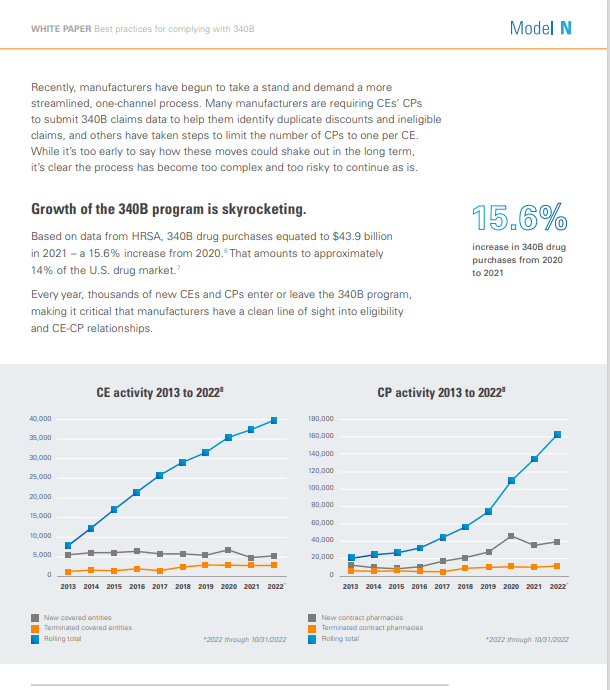
Changes to the 340B program have led to an increase in the number of covered entities and contract pharmacies, as well as more stringent financial and legal implications for price errors.
For pharmaceutical manufacturers that participate in the 340B program, effective management of pricing, rebates, and chargebacks is crucial to protecting revenue streams and ensuring compliance. In this white paper, you’ll learn how you can improve your chargeback process, generate an audit trail, and ensure correct 340B pricing.
In this white paper we will:
- Discuss important changes to the 340B Drug Pricing Program.
- Provide best practices and practical approaches for streamlining 340B program management.
- Explain how you can reduce the risk of revenue leakage and noncompliance.
Some of our customers:




Thank you for your interest in the Whitepaper: Best Practices for Complying with 340B
You might also be interested in



Preparing for Commercialization While You’re in Development
Learn how to position yourself in the market with innovative launch strategies and relationship building. VIEW webinar


Novartis and Model N: Partners in Revenue Management
Novartis has been a user of Model N’s revenue cloud for life sciences for over 15 years, as one of Model N’s founding clients. Over the years, Novartis has leveraged the Model N platform to automate pricing... VIEW case-studies


Maximize the Value of Innovative Commercial Contracts
Innovative contracting approaches are growing in popularity with pharmaceutical companies. They drive demand and sales, but they also come with potential pitfalls that can impact your company’s success. VIEW whitepaper
.
Start typing and press Enter to search
Contact Sales
Thank you for your interest in Model N. Please submit the form and our sales team will contact you within 24 hours.
Our Customers Include:
Thank you for reaching out.
We look forward to responding within one business day.








